Homework is to complete the Energy Diagram lab from class, as well as the two math worksheets also handed out. The first worksheet is Temperature scales (converting between Fahrenheit, Celsius and Kelvin scales - if you need help, look in textbook 5.7 or online); the second worksheet is about specific heat capacity calculations. The answers are linked at the bottom of the page. As you know, there may be errors. These worksheets should be finished by next Monday, but be sure to get going now so you can ask questions on Thursday if needed.

The textbook sections to cover is chapter 10.4-10.5.
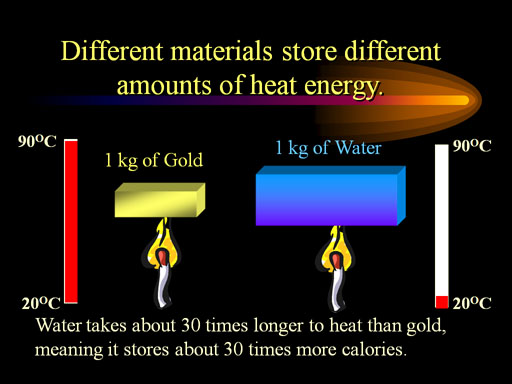
Q = mcΔT
Q is the energy in joules
m is mass in grams
c is the specific heat (from the chart) and the unit is J/g°C (the textbook uses s instead of c)
ΔT is the change in temperature in °C. It could be positive or negative.
Answers to Energy Diagram Lab, Temp. Scales, and Specific Heat ***If/when you find errors, add comments.

Question 1 of the Specific Heat worksheet I'm getting 240.16 JK .
ReplyDeleteQuestion 10, 118 F is not 65.6 C, it is 48 F. Sorry for the lack of degree symbols.
DeleteBut I'm getting a bad answer...So...
DeleteOn the study guide for the Specific Heat worksheet, it says that 5,007/1.184 is 124.6. It is really about 1,196.
ReplyDelete