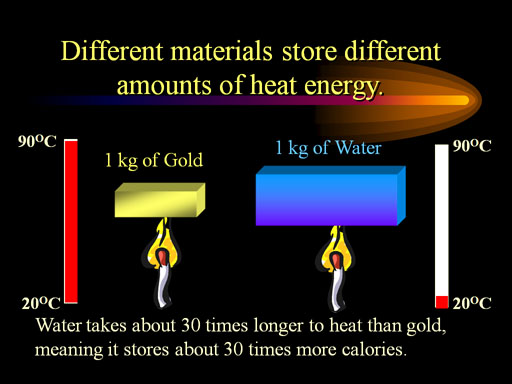
For Review:
Read textbook 10.6, work the self-check
and answer questions 22-28 and 29-31. ( Also review key terms and summary on page 317). My answers will be below...soon. Also, could you email me to let me know how you are doing on this latest bit of math (Q=mc(delta)T and the new stuff). And then back to Hank:
Last bit of homework, this worksheet: Bond Energies but just do problems 1-3, and optional, if you remember Lewis diagrams from middle school physical science, try 6 & 7. Answers here https://drive.google.com/file/d/0B07s3-bcUr7tdzBnNW9CdEJvaDA/edit?usp=sharing
************************************Weird Historical Twist*************
In the late 1940s, German theoretical physicist Arnold Sommerfeld, having previously written a series of books in physics: mechanics (1943), electrodynamics (1948), optics (1950), etc., was asked why he had never written a book on thermodynamics? The following is his humorous and frequently quoted answer: [8]
“Thermodynamics is a funny subject. The first time you go through it, you don't understand it at all. The second time you go through it, you think you understand it, except for one or two small points. The third time you go through it, you know you don't understand it, but by that time you are so used to it, it doesn't bother you anymore.”
In an odd twist of fate to this quote, in April of 1951, while in the midst of writing a book on thermodynamics (Thermodynamics and Statistical Mechanics), and having been nominated 81 times for the Nobel Prize (more than any other physicist), but not yet having won, Sommerfeld was killed from injuries after a traffic accident while walking his grandchildren. The book was published post-humorously the following year. [9]