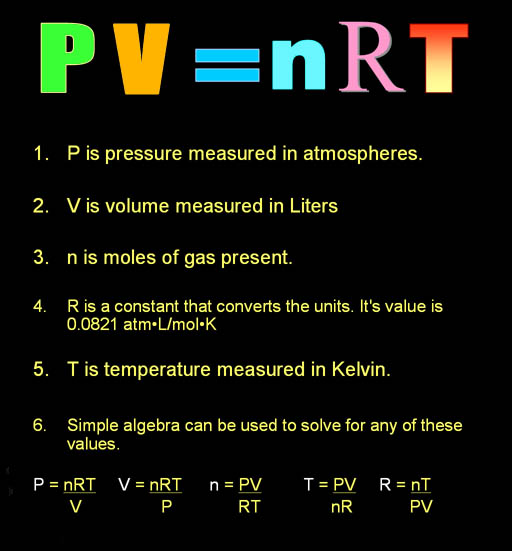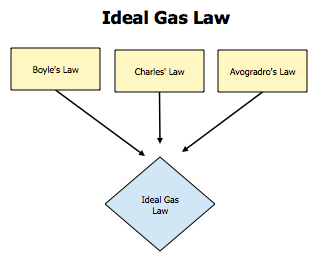
Homework is to complete the two worksheets handed out in class. Answers
Missing worksheet?
http://www.csun.edu/~jte35633/worksheets/Chemistry/14-3CombinedGasLaw.pdf
http://www.csun.edu/~jte35633/worksheets/Chemistry/14-4IdealGasLaw.pdf
And here is some reading on gases: http://www.chemistryland.com/CHM130S/12-Gases/Gases.htm
STUDYING FOR THURSDAY'S TEST: I've decided to limit the test to the material in chapters 11 -12, so review the Key Terms and Summary at the end of each chapter, and especially the worksheets. Any questions? If you would rather delay the previous homework till spring break in order to study for the test, that's OK with me. I will not be assigning additional homework for spring break.
| And neither of them are wearing safety goggles. |